PUBLICATIONS
Recent publications highlighting cutting-edge organic photoredox radiolabeling technology from our laboratories.
Radiofluorination of (hetero)arenes via isotopic exchange
Given the prevalence of fluoro(hetero)arenes in active pharmaceuticals, fluorine isotope exchange provides a convenient method for radiotracer synthesis.
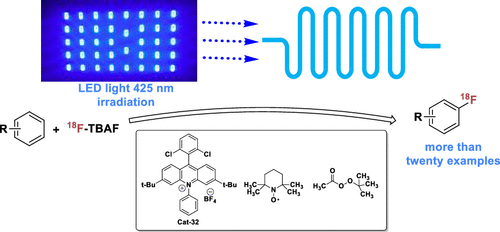
Arene C-H radiofluorination of arenes via isotopic exchange
We have reported an improvement on our C-H radiofluorination that uses a flow photoreactor and peroxide as a terminal oxidant.
Radiofluorination of alkoxy(hetero)arenes via SNAr
We have demonstrated that phenol derivatives can undergo radiofluorination via nucleophilic aromatic substitution (SNAr) via organic photoredox catalysis.
Radiocyanation of (hetero)arenes via demethoxylation
The [11C] radioisotope can be introduced in aromatic compounds via an organic photoredox-catalyzed nucleophilic aromatic substitution of methoxyarenes.
Direct C-H radiofluorination of (hetero)arenes
In seminal work from our laboratories, we demonstrated that (hetero)aromatics readily undergo radiofluorination via organic photoredox catalysis. This advance means that no prefunctionalization of the aromatic is required.
C-H Radiocyanation of (hetero)arenes
The direct introduction of [11C] via C-H radiocyanation of (heater)arenas was accomplished via organic photoredox catalysis.